Navigating the New Amendments to the EU In Vitro Devices Regulation (IVDR)
The European Union has recently voted to adopt several key amendments to the In Vitro Devices Regulation (IVDR), aiming to address some of the persistent challenges in implementing this comprehensive new framework. In this article Dave Smart, a respected precision medicine expert at Diaceutics, provides his insights into these significant changes and their implications.
Extended deadlines for compliance
One of the most notable changes is the extension of the transition period for different classes of in vitro diagnostic devices to achieve full compliance with the IVDR. Here are the updated deadlines:
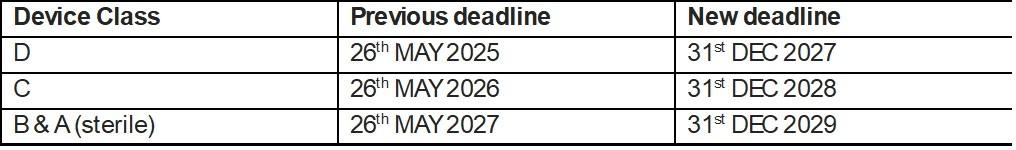
These extensions are crucial, especially for Class C devices, which encompass companion diagnostics - a cornerstone of precision medicine. It's important to note that this extension only applies to legacy devices that were:
- compliant under the previous In Vitro Devices Directive (IVDD),
- have not undergone significant changes in design or intended use, and
- do not pose an unacceptable risk to patient health or safety.
Additionally, manufacturers must implement a quality management system (QMS) by 26th May 2025.
Impact on Laboratory-Developed Tests (LDTs)
The amendments do not explicitly address laboratory-developed tests (LDTs). However, it is assumed that the deadline for compliance with Article 5.5(d)—which requires demonstrating that the specific needs of the target patient population cannot be met by existing market products - will also be extended.
Addressing potential diagnostic shortages
The EU acknowledges that even with extended deadlines, manufacturers might face challenges that could result in a shortage of critical diagnostic tools. To mitigate this, the amendments now mandate that manufacturers provide prior notice of any anticipated shortages of in vitro diagnostics to relevant stakeholders, including downstream economic operators. This proactive measure aims to ensure continuity in the availability of essential diagnostics.
Phased implementation of the Eudamed database
Another significant change involves a phased approach to the implementation of the Eudamed database. Manufacturers are now required to provide information to the five existing modules before the remaining modules are completed. This phased implementation is expected to be in place by Q4 2025. The EU will continue to monitor the rollout of the IVDR and the related Medical Device Regulation, suggesting that further amendments could be on the horizon.
Conclusion
These amendments represent a thoughtful response by the EU to the complexities of transitioning to the new IVDR framework. By extending deadlines, addressing potential shortages, and phasing the implementation of critical systems like Eudamed, the EU aims to facilitate a smoother transition for manufacturers and maintain the availability of vital diagnostic tools. As always, Diaceutics remains committed to supporting our partners in navigating these regulatory changes and advancing precision medicine.
