Radioligand therapy: How is this transforming radiotherapy into a precision medicine treatment
Radiation therapy is one of the first non-surgical cancer treatments. It was established in the late 19th century and has remained an integral part of cancer care ever since. Although radiation has a high potency for killing tumor cells, it also causes significant damage to the surrounding healthy tissue, resulting in severe side effects.
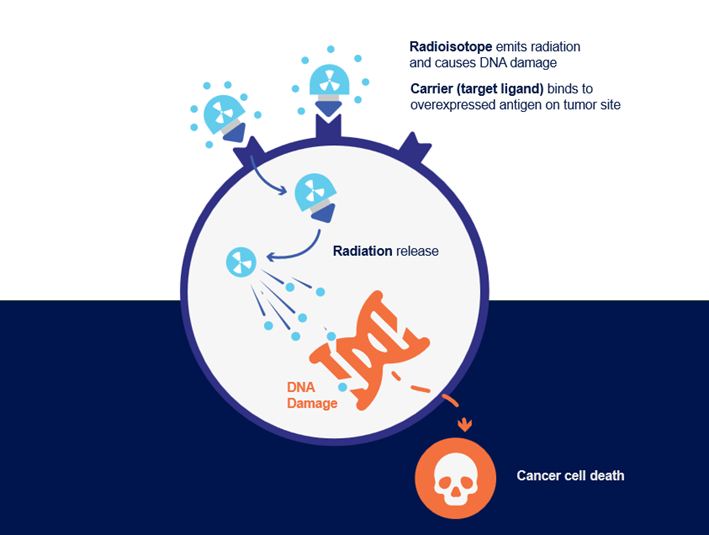
The recent development of radioligand therapy (RLT) has provided the therapeutic upgrade that medicine was hoping for. RLT is a form of precision nuclear medicine. Administered as intravenous infusion, it delivers radioactive particles and their detrimental effects directly to tumor sites by binding to target molecules overexpressed on tumor cells or the tumor micro-environment (Figure 1). This limits off-target effects and enables access to tumor sites that would be difficult to irradiate externally, setting RLT apart from traditional radiation treatments.
Despite all the benefits, clinical implementation of RLT is still facing numerous challenges related to regulatory policies, awareness, and availability of the treatment, which need to be tackled in order to allow eligible patients to receive treatment.
Approved RLTs and novel strategies
(Radio) Theranostics refers to a personalized treatment where paired radiopharmaceuticals are used for diagnostic imaging and selective treatment of a patient. It’s utilization of radiotracers, composed of radiation particles attached to a carrier molecule which will bind specifically to its target on tumor, allows for the initial confirmation of target overexpression on tumor sites followed by RLT application. This approach facilitates earlier diagnosis, better stratification of patients and subsequently, better outcomes (1,2).
The first two ground-breaking RLTs were Lutathera and Pluvicto for somatostatin receptor (SSTR) positive mid-gut neuroendocrine tumors (NETs) and prostate-specific membrane antigen (PSMA) positive prostate cancer respectively (3-6). Considering the unique potential of RLT to create numerous combinations of radioactive isotopes and to target molecules tailored to specific tumor types, many more RLTs are both now in development and likely to come to the market in the near future.
RLTs are already being tested in clinical trials for other SSTR expressing tumors beyond NETs, such as small-cell lung cancer, GBM and meningioma (1,7). Novel strategies targeting PSMA for prostate cancer therapy are also in clinical trials, comparing beta-emitting lutetium (177Lu)-labeled RLT to alpha-emitters such as actinium (225Ac), or combining both (2,7,8, 13, 16).
New targets like GRPR, EGFR-cMET, IGF-1R, FRα, NTSR1, CA9/CAIX, LAT1/2, GPC3 etc. are also being investigated in various tumor types (7, 10, 12,13, 15, 16). Expanding beyond tumor cells, novel RLTs are targeting cancer-associated fibroblasts and the stromal matrix in tumor microenvironments (1, 2, 7, 11, 17). Combining RLTs with already established systemic treatments such as chemotherapy, immunotherapy, or agents inhibiting DNA repair like PARP inhibitors, is another emerging approach being tested with the aim to improve patients’ outcomes (1,2).
As RLTs are becoming relevant therapeutic options for a growing number of patients, the current success of approved treatments is expected to drive further development of novel targeting agents in order to improve cancer detection, patient stratification and personalized treatments.
Current challenges
Medical innovations, despite their clinical benefits, usually come with some challenges and this holds true for RLTs as well (1).
- When it comes to RLTs, regulation is currently the name of the game, dictating access to approved treatments, clinical trials and radioisotopes. There is great variability across different countries with respect to local rules and regulations for RLT, so understanding the regulatory requirements at the country level and building towards uniformity is critical. The same holds true for reimbursement frameworks.
- Due to the fast radioactive decay, these treatments have a short shelf-life. This imposes tremendous time pressure on manufacturers and clinicians to produce, transport and administer treatment within a few days. Ensuring a consistent supply of radioisotopes can also be challenging.
- Currently there is a limited number of healthcare professionals properly trained in RLT, highlighting not only a strong need for more physicians specialized in nuclear medicine, but also for multidisciplinary training programs on RLT in general. Implementation of RLTs requires procedural adjustments and further integration of nuclear medicine with oncology.
- With the growing number of patients, medical institutions will need to implement some structural adjustments in order to provide sufficient capacity for inpatient care, and to address additional requirements related to the equipment and storage for radioactive materials.
All these factors are currently restricting the use of the RLTs to a small number of specialized centers within a few countries.
Improving patient selection and outcomes: future perspectives.
Currently the objective response rate (ORR) for patients receiving RLTs is approximately 40-50%(3-6). Targeted PET imaging is, at the moment, the gold standard for selecting eligible patients. It is well established for approved indications, especially PSMA-positive prostate cancer, and is further being introduced/optimized across several different biomarkers/indications.
One of the underlying reasons for the lack of consistent therapy response is heterogeneous expression of target antigens on tumor cells or acquired resistance due to loss of cell-surface target molecules under the selective pressure of toxic radiation (1). Patient response to RLT differs not only with respect to treatment efficacy, but toxicity as well, defined by administered dose of radioactivity, clinical characteristics, previous treatments and possibly genetic factors (1).
Additional (biomarker) assessments of target expression, as well as better understanding of individual genomic profiles and its impact on patient’s response to RLT might prove critical for better patient stratification and improved clinical outcomes.
Unlocking Radioligand therapy potential with Diaceutics:
Diaceutics can support and accelerate your RLT-related strategies through a number of different solutions.
Our Daily DXRX Signal can help identify patients who would be potentially eligible for RLT, especially those with rare cancers, such as gastroenteropancreatic (GEP)-NET.
When no functional imaging is possible, alternative biomarker testing approaches can be considered (18). Diaceutics can support diagnostic partner selection for the development of complementary molecular assays and the implementation of novel biomarkers into regular clinical practice.
Our experts are always happy to assist with feasibility studies, and our DXRX Data Repository has the largest global and US lab dataset, allowing us to capture a robust view of the patient journey over a wide range of indications, no matter how rare.
Contact us
References
- Radiotheranostics in oncology: current challenges and emerging opportunities - PMC (nih.gov)
- Carrier systems of radiopharmaceuticals and the application in cancer therapy | Cell Death Discovery (nature.com)
- Phase 3 Trial of 177Lu-Dotatate for Midgut Neuroendocrine Tumors | New England Journal of Medicine (nejm.org)
- Novartis Lutathera® significantly reduced risk of disease progression or death by 72% as first-line treatment for patients with advanced gastroenteropancreatic neuroendocrine tumors | Novartis
- Lutetium-177–PSMA-617 for Metastatic Castration-Resistant Prostate Cancer | New England Journal of Medicine (nejm.org)
- Novartis radioligand therapy Lutathera® FDA approved as first medicine specifically for pediatric patients with gastroenteropancreatic neuroendocrine tumors | Novartis
- Novartis Pipeline | Novartis
- Development Pipeline | Bayer Global
- Bicycle Therapeutics - Pipeline - Bicycle Therapeutics
- Pipeline - Telix Pharmaceuticals
- Lilly to Acquire POINT Biopharma to Expand Oncology Capabilities into Next-Generation Radioligand Therapies | Eli Lilly and Company
- AstraZeneca to acquire Fusion to accelerate the development of next-generation radioconjugates to treat cancer
- Fusion Pipeline - Fusion Pharma - Pipeline
- Bristol Myers Squibb - Bristol Myers Squibb Completes Acquisition of RayzeBio, Adding Differentiated Actinium-Based Radiopharmaceutical Platform (bms.com)
- Pipeline Overview - RayzeBio
- ITM Radiopharma: Research & Development (itm-radiopharma.com)
- Pipeline|PEPTIDREAM INC ペプチドリーム株式会社
- ESMO Interactive Guidelines
